Press-room / news / Science news /
Potent painkiller from spider venom
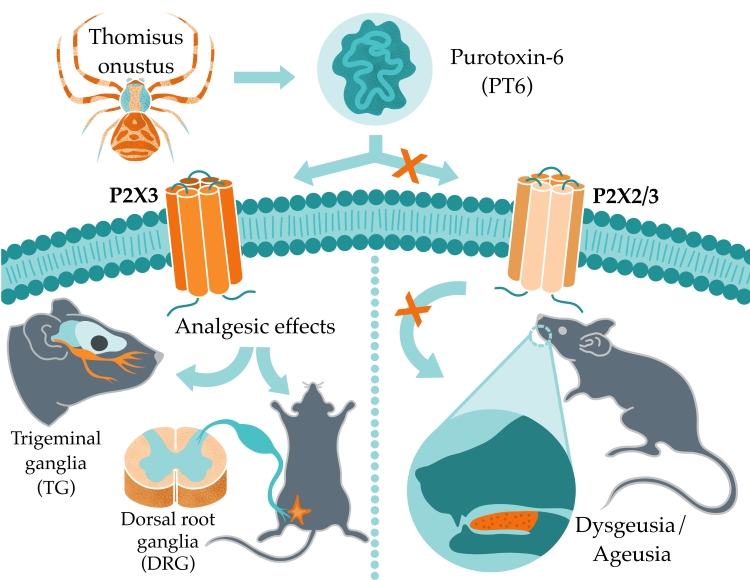
A whole family of peptides with completely unexpected activity has been discovered in spider venom. These peptides inhibit mammalian purinergic receptors with high affinity and selectivity. A peptide called purotoxin-6 (PT6) from the venom of the crab spider Thomisus onustus inhibits P2X3 receptors, an important pharmacological target in a number of pain syndromes and chronic cough. PT6 has a compact fold and exhibits a potent analgesic effect in animal models of osteoarthritis and trigeminal neuralgia. At the same time, unlike small-molecule P2X3 ligands that are being developed as drugs, purotoxin does not cause dysgeusia, i.e., distortion of the sense of taste. Research on purotoxins began at the Institute of Bioorganic Chemistry some 20 years ago under the supervision of Academician Eugene Grishin and was successfully continued by Alexander Vassilevski. The results, unique on a global scale, were published in Molecular Therapy.
Artwork by Anastasiia Samoukina
Ionotropic purinergic receptors (P2X) are ion channels activated by extracellular ATP. The P2X3 isoform is predominantly expressed in sensory neurons of the dorsal root ganglia and trigeminal ganglia and plays an important role in chronic pain, making it an attractive target for drug development. P2X3 has been implicated in pain in a variety of conditions, including osteoarthritis, irritable bowel syndrome, cystitis, as well as neuropathic pain, migraine, and chronic idiopathic cough. Several small-molecule P2X3 antagonists have been developed; some are in clinical trials and one has recently been approved in Japan, Switzerland, and the European Union for the treatment of refractory chronic cough. A major drawback of most of the known P2X3 inhibitors is their side effects. In particular, the low selectivity for P2X3 compared to the P2X2/3 heteromer causes dysgeusia, i.e., distortion of taste, which is a major problem encountered during clinical trials.
Our work involved several steps. Back in the early 2000s, we isolated and characterized purotoxin-1 (PT1), the first selective high-affinity peptide ligand of P2X3. We then obtained cDNA libraries of venom glands from various spiders. It turned out that PT1 homologs form a large family of purotoxins (Figure 1A). We selected the smallest homolog, called PT6, which could be obtained in a bacterial expression system at a high yield. Here, the concept of natural venoms as combinatorial libraries played the key role: toxins form libraries of related compounds, similar to those used by pharmaceutical companies. The natural library of purotoxins suggested a “minimalistic” structure of PT6. Using NMR spectroscopy, we studied the spatial structure of PT6 in solution; it presents a shortened β-hairpin loop and one disulfide bond is missing compared to PT1 (Figure 1B, C).
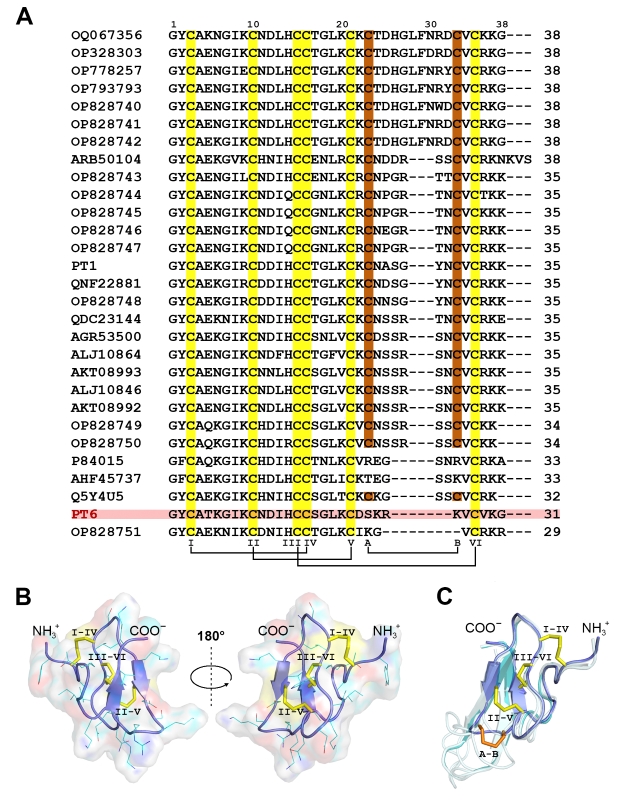
Figure 1. Structure of purotoxins. (A) Combinatorial library of purotoxins. The amino acid sequence of PT6 is highlighted. The arrangement of disulfide bridges is shown at the bottom. Cysteine residues that form a disulfide bond, which is absent in PT6, are highlighted in brown. (B) Three-dimensional structure of PT6 (PDB ID: 6F61). (C) Comparison of the structures of PT6 and PT1 (PDB ID: 2KGU), as well as homology-based models of other purotoxins. The polypeptide chain of PT6 is colored purple, PT1 is colored blue. Disulfide bridges are shown, colored as in panel A.
We confirmed that PT6 retained specific activity towards P2X3 using both recombinant receptors expressed in cell lines and isolated neurons from dorsal root and trigeminal ganglia. Importantly, PT6 inhibited P2X3 with high affinity (EC50 = 8 nM) but was not active on P2X2 or P2X2/3 heteromers even at micromolar concentrations (Figure 2).
PT6 exhibited antinociceptive activity in non-specific but widely used animal tests such as the formalin test, CFA test, and writhing test. We further confirmed the selectivity of PT6 using P2rx3-knockout mice, which lack a functional P2X3 receptor; PT6 lost its analgesic activity in the knockout mice. We then examined the activity of PT6 in a number of animal models of pain syndromes with known or suspected involvement of P2X3, including models of osteoarthritis and trigeminal neuralgia. In all cases, PT6 was highly effective. Moreover, the effective doses of purotoxin (0.01–0.1 mg/kg) were several orders of magnitude lower than the doses of commonly used analgesics, including nonsteroidal anti-inflammatory drugs and opioids. Finally, we tested whether PT6 affects taste perception in comparison with small-molecule P2X3 inhibitors currently under development by pharmaceutical companies. It turned out that, unlike small molecules, PT6 does not cause dysgeusia.
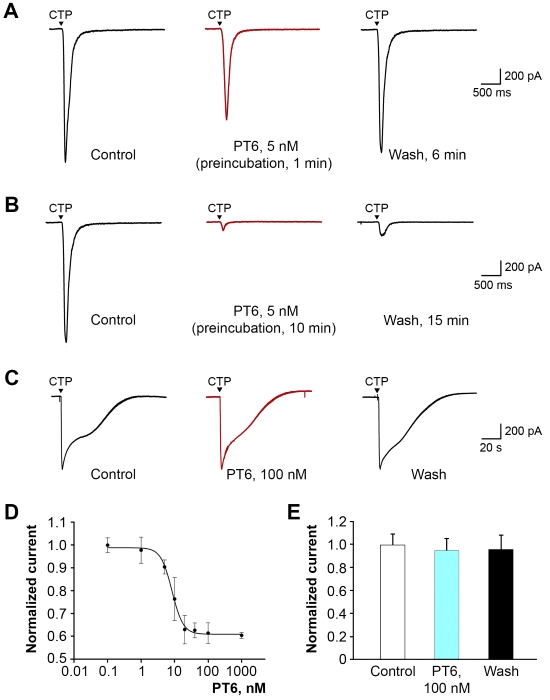
Figure 2. Specific activity of PT6 on P2X3 receptors. (A and B) Current recordings in CHO cells expressing P2X3 in response to the application of agonist (100 μM CTP). With a short-term (1 min) preincubation (A), the effect of 5 nM PT6 was small and completely reversible. With a long-term preincubation (B), the current was significantly suppressed, up to complete inhibition, which was only partially reversible. (C) Current recordings in cells expressing P2X2/3 heteromers. (D) The dose-response curve for short-term incubation is described by the Hill equation; EC50 = 8.4 ± 0.5 nM, h = 2.7 ± 0.3. (E) Responses recorded for P2X2/3 heteromers.
Most of the work was carried out at the Institute of Bioorganic Chemistry of the Russian Academy of Sciences, by various departments. Colleagues from other organizations and scientific centers of Russia played an important role in the research:
- "Future Analgesics", a resident of "Skolkovo", is developing an innovative analgesic based on purotoxin
- purotoxin mode of action was studied at the Institute of Cell Biophysics of the Russian Academy of Sciences
- the activity of purotoxins on the trigeminal nerve was explored at the Institute of Fundamental Medicine and Biology, Kazan Federal University
- knockout mice were obtained at the Center for Precision Genome Editing and Genetic Technologies for Biomedicine, Institute of Gene Biology of the Russian Academy of Sciences,
- analgesic activity tests were conducted on knockout mice at the Zakusov Institute of Pharmacology,
- the activity of purotoxins in a neuralgia model was studied at the Institute for Biomedical Problems of the Russian Academy of Sciences.
march 6


