Press-room / news / Science news /
Synthesis of Substituted 1,2,4-Triazole-3-Thione Nucleosides Using E. coli Purine Nucleoside Phosphorylase
Scientists from the departments of biotechnology and structural biology (IBCH RAS) and Institute of the Chemistry of Plant Substances (Uzbekistan), and D. I. Ivanovsky Institute of Virology synthesized a series of substituted 1,2,4-triazole-3-thione nucleoside analogs and tested their antiviral activity against herpes simplex virus.
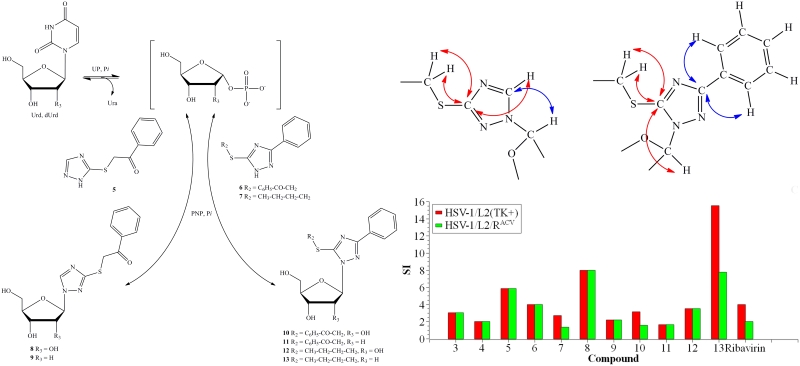
1,2,4-Triazole derivatives have a wide range of biological activities and finding new nucleosides based on it is a topical task. Three compounds from a series of synthesized mono- and disubstituted 1,2,4-triazole-3-thione derivatives were found to be substrates for E. coli purine nucleoside phosphorylase. For ribosides and deoxyribosides produced by enzymatic synthesis, it has been shown that the addition of carbohydrates to mono- and di-substituted 1,2,4-triazole-3-thiols occurs at different nitrogen atoms. All synthesised nucleosides and heterocyclic bases were tested for cytotoxicity and activity against herpes simplex virus 1. Compared to the antiviral drug ribavirin, the selectivity index for the two nucleosides was significantly higher. It was also found that as the lipophilicity of the compounds studied increased, both their activity and toxicity increased. The results are published in the Biomolecules (IF 4.8).
july 4, 2024