Laboratory of Biomedical Materials
The Group deals with development and study of biodegradable materials (scoffolds) based on natural and synthetic polymers for tissue engineering and regenerative medicine. These biomaterials could be designed in different forms, such as nano- and microfibers, hydrogels, microbeads (microcarriers for cell culture). To study in vitro cytotoxicity and other properties, various cell cultures are used, including fibroblasts, osteoblasts-like cells, mesenchyme stem cells etc.). Moreover, the Group is engaged in evaluation of anticancer drug delivery systems, namely nanoparticles, micelles, liposomes, polyelectrolyte nanocontainers etc.
On the other hand, novel 3D in vitro models based on multicellular tumor spheroids have been developed. These tumor spheroids are promising for evaluation of mechanisms of various anticancer therapies (chemotherapy, photodynamic therapy etc.) mechanisms as well as for screening of novel anticancer drugs and drug delivery systems directly before preclinical tests. This approach allows to reduce costs of preclinical tests and to minimize a number of experimental animals used for this purpose.
The Laboratory cooperates with the laboratories of the Institute, as well as with University of Liege (Belgium), Polytechnical University of Nancy ENSAIA-INPL (France), University of Strasbourg (France), Queen’s University of Kingston (Canada) etc.
The group was founded as an independent division in 2017, having separated from the Laboratory of Polymer for Biology.
The Group is engaged in development some biodegradable matrices for regenerative medicine (Fig. 1), anticancer drug delivery systems (Fig. 2) (3D in vitro models based on tumor spheroids, microencapsulated tumor spheroids ( Fig. 3), tumor spheroids prepared from monolayer cell culture using RGD-peptides (Fig. 4), spheroids from tumor and normal cells generated using RGD-peptides (Fig. 5)).
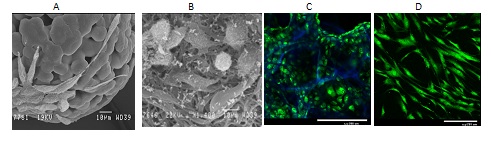
Fig. 1. Growth of mouse fibroblasts L929 on biodegradable poly-L.D-lactide microcarriers (A), microfibers (B), in macroporous hydrogels based on chitosan and hyaluronic acid (С), and human mesenchymal stem cells on chitosan films treated with DC discharged plasma (D). Cells are stained with vital Сalcein AM dye (in green), while the matrix structure is visualized with DAPI dye (in blue). SEM (A,B) and laser confocal microscopy (С,D).
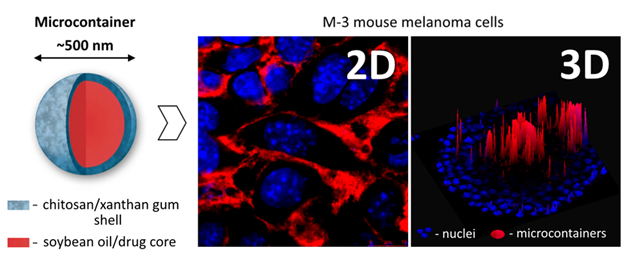
Fig. 2. Polysaccharide microcontainers for anticancer drug delivery and microcontainer accumulation within M-3 mouse melanoma cells in 2D (monolayer culture) and 3D (spheroids) in vitro models.
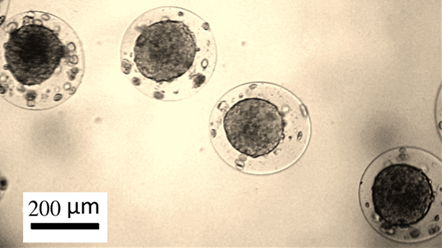
Fig. 3. Tumor spheroids from human breast adenocarcinoma MCF-7 cells generated within biocompatible alginate-chitosan microcapsules.
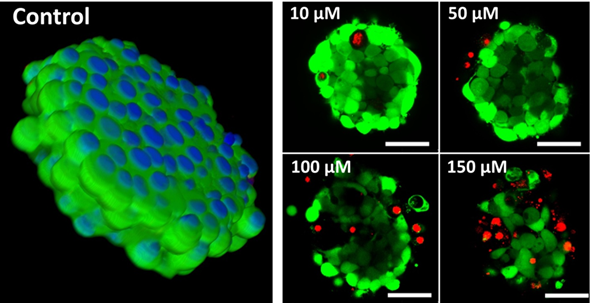
Fig. 4. Generation of tumor spheroids by RGD-induced cell self-assembly after adding RGD-peptide directly to monolayer cultures.
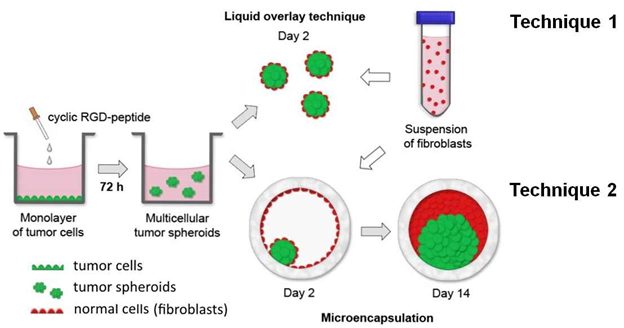
Fig. 5. Two techniques (LOT и Microencapsulation) to generate spheroids from tumor and normal cells using RGD-platform.
| Fullname | Position | Contacts |
|---|---|---|
| Elena Markvicheva, D.Sc | pr. r. f. | |
| Roman Akasov, Ph.D. | s. r. f. | |
| Oksana Selina, Ph.D. | r. f. | |
| Maria Drozdova | r. f. | |
| Anastasia Gileva | j. r. f. | |
Previously worked here | ||
| Anna Khovankina | ||
| Biryukova V.N. | ||
| Mamedova A.R. | ||
| Terehova V.V. | ||
| Smirnov I.V. | ||
 Loading...
Loading...Scientific projects
 Loading...
Loading...Elena Markvicheva
Russia, Moscow, Ul. Miklukho-Maklaya 16/10 — On the map
 Loading...
Loading...
