Press-room / news / Science news /
“Molecular portraits” characterized functional states of TRPV ion channels
TRPV ion channels realize a huge variety of functions in the human body participating in the temperature and pain sensation, cell division, calcium uptake. Researchers from IBCh RAS and Columbia University analyzed the structure of the key TRPV domain – the ion conducting pore. Using the original “dynamic molecular portrait” approach, they identified three major states of the pore that are common for all TRPVs, called α-closed, π-closed, and π-open. It was shown that the α-closed state is the most hydrophobic and always nonconducting. While the π-closed one is less stable and can easily transit to the open state, which has favorable hydrophobic properties for the ion conduction. The results were published in Communications Chemistry.
TRPV family of ion channels combines membrane proteins noticed by the huge variety of functions in the human body. Among others, they act as molecular “detectors” responsible for pain and temperature sensation (nociception), participate in the cell division, calcium uptake and so on. Such a functional diversity makes TRPVs a promising target for a wide range of newly developed drugs, primarily analgesic and anti-cancer compounds. This demands a detailed investigation of the TRPV functioning mechanisms, both in normal and pathological conditions. Valuable information in this direction is provided by the 4 experimental methods of structural biology, mainly by the cryo-electron microscopy, that can solve the atomic resolution structures of the ion channels in various functional states. However, static structures often do not provide comprehensive information about the ensemble of protein states. More sophisticated analysis is required to reveal the dynamic characteristics of such states and transitions between them.
Researchers from the Laboratory of Biomolecular Modeling of IBCh RAS together with the colleagues from Columbia University (New York, USA) analyzed the structure of the key TRPV domain – the ion conducting pore. The original molecular modeling-based approach called “dynamic molecular portrait” (DMP) was used to reveal fine details of the pore structure. The method mainly focuses on the analysis of spatiotemporal physico-chemical properties of molecular surfaces and intermolecular interactions. One of the DMP methods used in the study is the building of 2D projections (maps) depicting the distributions of molecular surface properties. Figure 1 shows an example of such a map with the distribution of hydrophobic/hydrophilic properties on the surface of the closed TRPV3 pore. As part of this work, the mapping method was developed and implemented as a publicly available web tool “Molecular Surface Topography” (MST) (https://model.nmr.ru/cell).
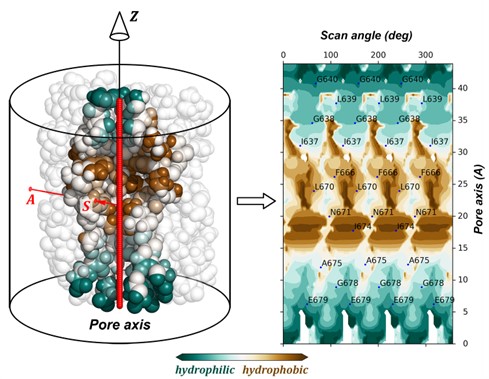
Fig.1. The method of building a 2D cylindrical projection (map) depicting the distribution of hydrophobic/hydrophilic properties (Molecular Hydrophobicity Potential, MHP) on the surface of the closed TRPV3 pore. Left: the molecular surface is shown as a superposition of Van der Waals spheres – protein atoms colored according to the MHP values on their surface (brown – hydrophobic areas, blue green – hydrophilic ones), around the latter, the projection cylinder is placed. The pore axis is shown with a thick red line, AS is the projection trace ray, Z is the cylinder axis. Left: the resulting map with MHP distribution on the pore surface. More details are given at https://model.nmr.ru/cell.
Using earlier obtained conformational ensembles of the three TRPV channels: TRPV1, TRPV3 and TRPV6, the authors showed, that only three major states of the pore exist, that are common for all TRPVs, called as α-closed, π-closed, and π-open (Figure 2). At the activation gate – the pore region controlling the channel conduction - α-closed state is the most hydrophobic and has the most favorable residue packing, what makes this gate state the most stable and always non-conductive. The gate of π-closed state is less hydrophobic and has unfavorable intermolecular contacts that, apparently, allows the channel to transit into the open state without significant energy barrier. In turn, the π-open pore forms poor hydrophobic environment favorable for the ion and water conduction. The DMP approach can also be used for structural and functional classification of other types of ion channels. The work was supported by RSF grant 23-14-00313 and published in Communications Chemistry.
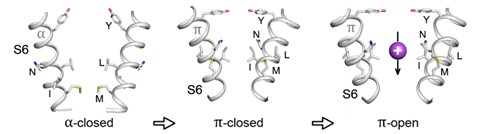
Fig.2. General gating scheme of TRPV illustrating consecutive transition states of the gate: α-closed → π-closed → π-open. Fragments of the pore-forming helices S6 are shown in cartoon representation (only two of four protein subunits are shown for clarity), gate lining residues are shown as sticks and marked, a purpure sphere shows the cation passing the open gate.
june 28, 2024