Press-room / news / Science news /
Immune system regulation for nanoparticle drug delivery. Breaking the endless cycle in nanomedicine
The journey of discovery in scientific research sometimes follows a familiar path: discover, admire, investigate, disappoint, and forget. Nevertheless, in some disciplines, it seems repeating many times. One of such cycles in the field of immune system blockade by nanoparticles is analysed in a recent article published in Nature Communications journal. Scientists from the Institute of Bioorganic Chemistry, Uppsala University and Boston University propose that advancements in nanomaterial development may finally disrupt this cycle, potentially introducing the method of macrophage blockade into clinical practice to improve cancer therapy.
Macrophages pose a primary barrier when using nanoparticles for therapy. Upon intravenous injection of nanoparticles, these immune cells swiftly recognize and eliminate them within minutes, resulting in less than 1% of the nanoparticles reaching the tumor site. This significantly diminishes therapeutic efficacy while increasing toxicity to normal organs. To solve this problem, macrophages can be distracted and saturated by pre-injection of non-toxic and biodegradable nanomaterials. After that injected therapeutic particles will longer circulate in the blood and have better probability of reaching targeted tissues in the body. This method is called “macrophage blockade” (Figure 1).
The concept of macrophage blockade has undergone several cycles of interest over the years. The first studies on the macrophage blockade date back to the era of early research on the physiology of the immune system in the 1910s. Scientists found that carbon-based inks are differently eliminated from the blood, depending on what had been introduced into animals. This early era showed similarity of the blockade mechanisms among different animals from frogs and fishes to birds and mammals. However, these early endeavors faltered due to inconsistent particle compositions and the use of toxic nanoparticles like radioactive thorium for the blockade.
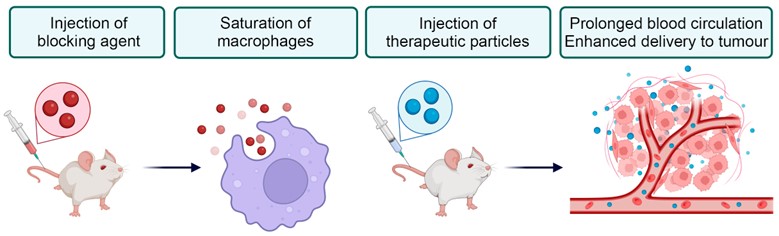
Figure 1. General concept of the macrophage blockade. Blocking particles (red) are eliminated by macrophages and saturate them. Subsequently administered therapeutics particles (blue) avert macrophage recognition, have longer blood circulation and better accumulation in tumor.
A resurgence of interest in macrophage blockade occurred in the 1960s, accompanied by the establishment of its mechanistic basis. Biocompatible and biodegradable materials, such as gelatin and protein nanoparticles, emerged as promising candidates for the blockade. Early evaluations of the method in clinic show its potential effectiveness for human treatment. However, in the 1980s, nanomedicine overcome the revolution. Invention of a polyethylene glycol, a stealth polymer for nanoparticle coating and reducing immune recognition seemed more promising for the clinic. The macrophage blockade was forgotten for another 30 years.
A resurgence of interest in macrophage blockade occurred in the 1960s, accompanied by the establishment of its mechanistic basis. Biocompatible and biodegradable materials, such as gelatin and protein nanoparticles, emerged as promising candidates. Despite early clinical success, the invention of “stealth” polyethylene glycol-coated nanoparticles in the 1980s overshadowed macrophage blockade for decades. Several nanoparticles with polyethylene glycol coatings entered the clinical usage. However, the method still had some drawbacks: it was not universal, many nanomaterials could not be successfully modified with polymer, and experienced adverse reactions to the polymer components.
The reinvention of the macrophage blockade did not take long to wait. In 2014, a group of scientists from China introduced non-toxic liposomes for the macrophage blockade and showed MRI visualization and subsequent therapy of tumors in mice. In 2020, scientists proposed using aged erythrocytes for macrophage blockade. A variety of new blockade methods were emerged (Figure 2).
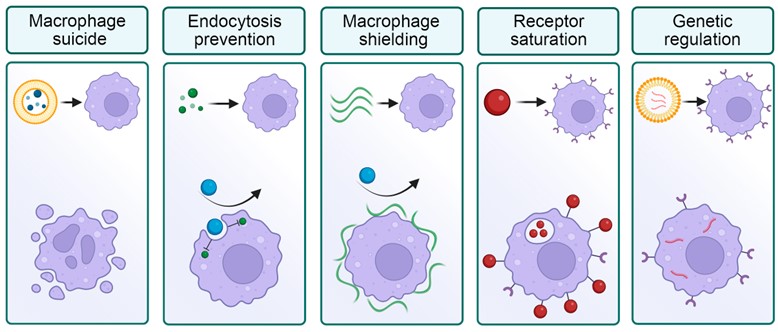
Figure 2. Selected strategies for the macrophage blockade. Left to right: elimination of macrophages with toxic compounds; prevention of nanoparticle processing by molecular inhibitors; shielding of macrophage surface; saturation of receptors by nanoparticles; genetic modification of cells.
In the perspective published in Nature Communications, authors performed a first meta-analysis of the efficacy of the macrophage blockade in prolonging nanoparticle half-life and enhancing tumor delivery. The method became safe, but the efficiency increased - by 40% in the "last cycle", potentially enabling effective tumor treatment. In addition, the authors analyze the properties that affect macrophage blockade and subsequent therapy. For example, larger particles induce stronger blockade due to faster cell binding. Moreover, blocking particles similar to therapeutic ones cause the most significant blockade. This is due to the depletion of specific opsonins in the blood – proteins, participating in nanoparticle recognition. Important, the method improves the delivery of targeted therapeutic particles, 10 times better than non-targeted ones. Blockade with modern nanomaterials lasts for several hours, after which the immune system fully recovers. This potentially opens doors for clinical usage of the method.
The study concludes by discussing barriers and future prospects in the field. Blood substitutes and lipid particles from intravenous nutrition are highlighted as promising blockade agents for clinical application. Furthermore, the potential extension of macrophage blockade to other biomedical domains, such as immunization and viral delivery, underscores its versatility and importance in advancing medical therapeutics.
june 14, 2024


