Press-room / news / Science news /
Unique CDR3 epitope targeting by CAR-T cells is a viable approach for treating T cell
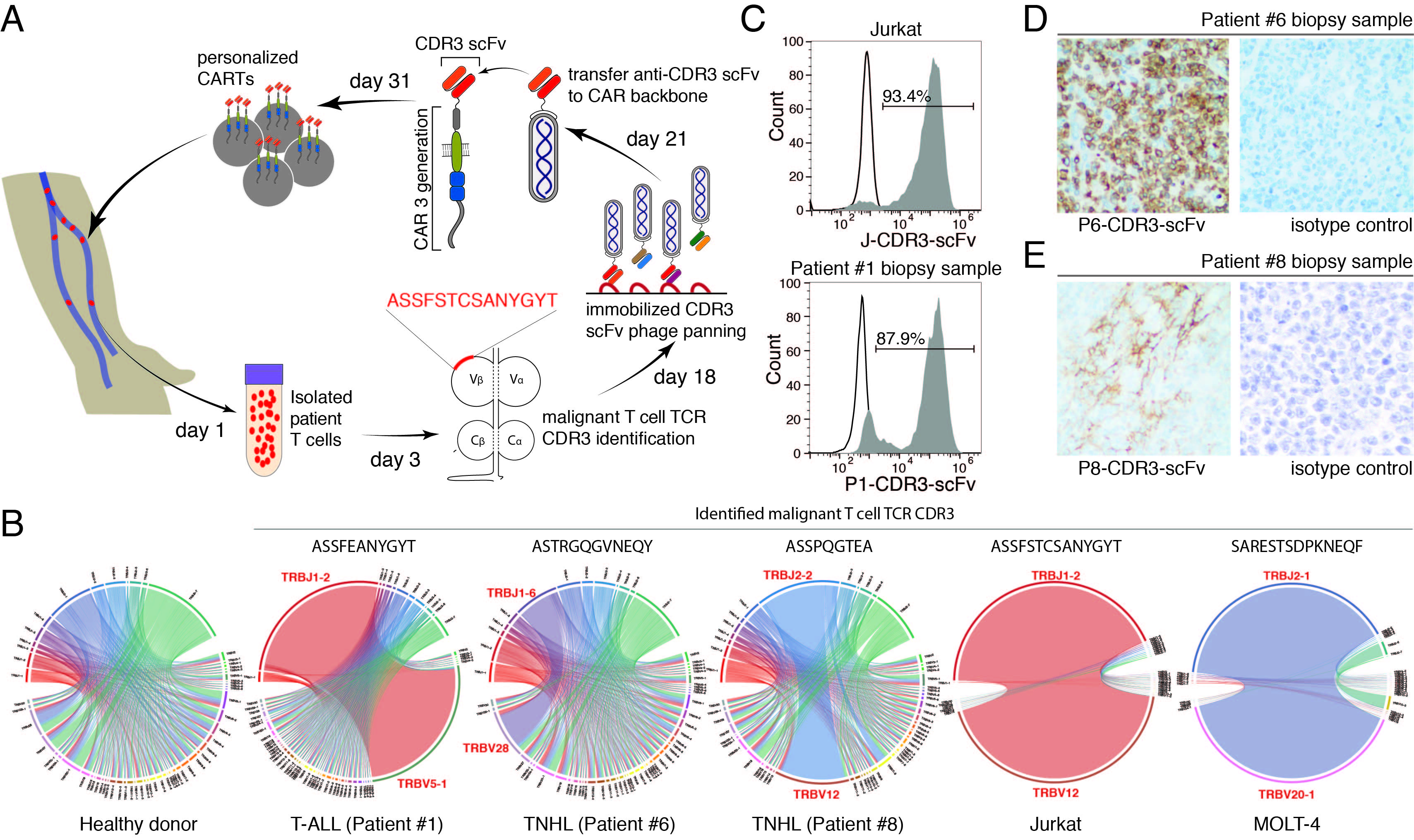
Efficient and specific removal of malignant cells is the ultimate goal of cancer therapy. The current rapid development of chimeric antigen receptor T cell (CAR-T cell or CART) therapy potentially provides high efficiency and allows long-term surveillance, which have greatly extended the frontier of leukemia treatment.
Efficient and specific removal of malignant cells is the ultimate goal of cancer therapy. The current rapid development of chimeric antigen receptor T cell (CAR-T cell or CART) therapy potentially provides high efficiency and allows long-term surveillance, which have greatly extended the frontier of leukemia treatment. We demonstrated for the first time that targeting the CDR3 regions of malignant T cell clones by cell therapy is a viable approach to eliminate leukemia cells. Due to its intrinsic uniqueness, the CDR3 region has historically been an intriguing target with much historical discussion. A few attempts have been made to use this region as the antigen for a cancer vaccine. Due to the clonal nature of leukemia cells and the uniqueness of a given CDR, targeting CDR3 should offer a few advantages not offered by targeting common antigens: 1. Lower “on-target, off-tumor” effect; 2. Minimized impact on a patient’s immune system during treatment; and 3. Great capacity for development as a personalized treatment that can be tailored to individual needs, as a percentage of patients do not respond well to existing therapies with common targets. In the current study, we validated the idea of using CARTs as targeting agents and observed excellent continuous efficacy as well as specificity. Combining CDR3 targeting with the CART approach provides a solution for a substantial portion of patients with T cell leukemia and lymphoma, with supposedly minimized side effects. The potential problems to be solved in the future include establishing efficient and streamlined good laboratory practice (GLP)-level CDR3 binder discovery and good manufacturing practice (GMP)-level personalized CART manufacturing and decreasing the financial burden for individual patients. However, these issues may be short-lived as technologies develop rapidly.
Nevertheless, after validation of this strategy to eliminate pathological T cells ex vivo and in vivo, we envisage this approach as a generally useful alternative and supplement to the popular approach of common antigen targeting to treat T cell malignancies, especially considering its safety. The work was supported by the Russian Scientific Foundation project No. 17-74-30019.
april 26, 2019

